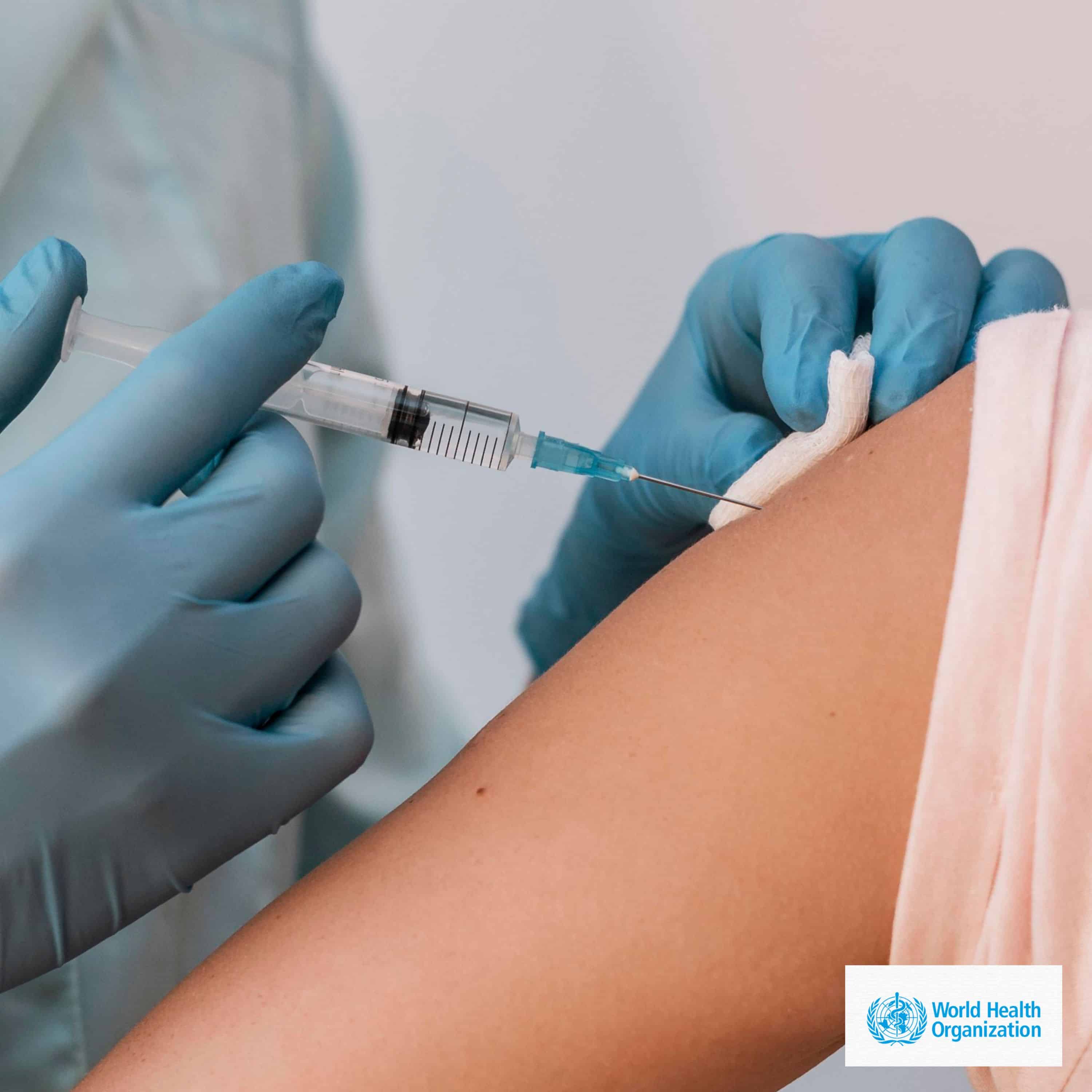
In a significant advancement in public health, the World Health Organization (WHO) has officially cleared the world’s first vaccine for mpox, formerly known as monkeypox, for use in certain regions. This landmark decision comes as health authorities worldwide strive to combat the spread of this viral disease, which has raised concerns due to its transmission patterns and potential health implications.
The vaccine, developed to provide immunity against mpox, has undergone rigorous evaluation through WHO’s “prequalification” and “emergency use listing” mechanisms. These processes are designed to assess the quality, safety, and efficacy of medical products, ensuring that they meet international standards before being deployed in affected areas. The approval signifies a crucial step in enhancing the global response to mpox outbreaks, particularly in regions where the disease has been endemic or where recent cases have surged.
Mpox, which is caused by the mpox virus, can lead to severe illness and complications, particularly in vulnerable populations. The disease primarily spreads through close contact with infected individuals, making vaccination an essential tool in controlling its transmission. The WHO’s endorsement of the vaccine is expected to bolster vaccination campaigns in areas most at risk, helping to curb the spread of the virus and protect public health.
Health experts emphasize the importance of vaccination as part of a comprehensive strategy to manage mpox outbreaks. Alongside vaccination efforts, public health initiatives will focus on raising awareness about the disease, promoting hygiene practices, and encouraging early diagnosis and treatment. The combination of these measures aims to reduce the incidence of mpox and mitigate its impact on communities.
Also Read: Wall Street Closes Higher as Inflation Data Fuels Expectations for Federal Reserve Rate Cut
The approval of the mpox vaccine has been met with optimism from health officials and organizations around the world. Dr. Tedros Adhanom Ghebreyesus, the WHO Director-General, expressed his support for the decision, stating, “This vaccine is a vital tool in our fight against mpox, and it will play a crucial role in protecting those most at risk.” He urged countries to prioritize vaccination efforts and ensure equitable access to the vaccine for affected populations.
In addition to the immediate public health implications, the introduction of the mpox vaccine also highlights the ongoing need for robust surveillance and research into emerging infectious diseases. The COVID-19 pandemic has underscored the importance of preparedness and rapid response to outbreaks, and the WHO’s approval of the mpox vaccine is a testament to the lessons learned from recent global health crises.
As countries prepare to roll out the vaccine, logistical challenges remain a consideration. Ensuring adequate supply chains, distribution networks, and public awareness campaigns will be critical to the success of vaccination efforts. Health authorities are encouraged to collaborate with international partners and organizations to facilitate the efficient deployment of the vaccine and maximize its impact.
Looking ahead, the WHO’s approval of the mpox vaccine represents a pivotal moment in the global health landscape. It not only provides a new tool for controlling mpox outbreaks but also reinforces the importance of international cooperation in addressing public health challenges. As the world continues to navigate the complexities of infectious diseases, the commitment to vaccine development and distribution will be essential in safeguarding health and preventing future outbreaks.
In conclusion, the WHO’s clearance of the world’s first mpox vaccine marks a significant milestone in public health efforts to combat this viral disease. With a focus on targeted vaccination strategies and comprehensive public health initiatives, there is hope for a brighter future in the fight against mpox and other emerging infectious diseases.